J Clin Oncol 28:15s, 2010 (suppl; abstr 3017)
Author(s):
H. A. Wakelee, S. N. Gettinger, J. A. Engelman, P. A. Janne, H. J. West, D. S. Subramaniam, J. W. Leach, M. B. Wax, Y. Yaron, P. Lara Jr.; Stanford University School of Medicine, Stanford, CA; Yale University School of Medicine, New Haven, CT; Massachusetts General Hospital Cancer Center, Boston, MA; Dana-Farber Cancer Institute, Boston, MA; Swedish Cancer Institute, Seattle, WA; Georgetown University Hospital Lombardi Comprehensive Cancer Center, Washington, DC; Park Nicollet Institute, St. Louis Park, MN; Summit Medical Group, Summit, NJ; Exelixis, South San Francisco, CA; University of California, Davis, Sacramento, CA
Abstract:
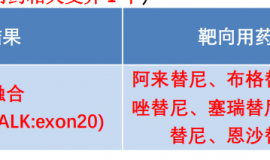
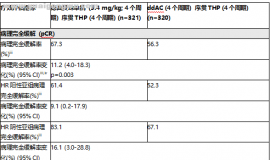
Background: MET and VEGF play important roles in the pathobiology of NSCLC, and are associated with resistance to E. XL184 is an oral, potent inhibitor of MET, VEGFR2, and RET, with antitumor activity in combination with E in NSCLC xenografts with acquired E resistance. Thus, XL184 + E may be effective in pts with acquired E resistance. Methods: Adults with previously treated advanced NSCLC received daily doses of XL184 + E in a 3 + 3 design using combination doses across 5 cohorts in 2 parallel arms (A and B). Objectives include safety, pharmacokinetics (PK), pharmacodynamics, and maximum tolerated dose (MTD) determination. RECIST response is assessed every 8 weeks. Pts with at least 2 post-baseline tumor assessments or pts who discontinued early for PD or AE were assessable for response. Results: 54 NSCLC pts have been enrolled in phase Ib, the majority had prior E. The dose levels were: arm B (75 mg XL184 + 150 mg E and 50 mg XL184 + 150 mg E (MTD) and arm A (75 mg XL184 + 100 mg E; 125 mg XL184 + 100 mg E; and 125 mg XL184 + 50 mg E). MTD determination of arm A is ongoing. Across all dose levels, 12 pts experienced at least 1 DLT: diarrhea, elevated AST, palmar-plantar erythrodysesthesia, mucositis, hypertension, hypokalemia, elevated lipase, and fatigue. Of 34 pts in safety database, most frequent frade 3/4 AEs were diarrhea (26%), fatigue (15%), dyspnea (12%), and hypoxia (9%). Preliminary PK analysis does not show evidence of DDI. Genotyping plasma tumor DNA from 28 pts revealed EGFR T790M mutations in 7 pts (25%). FISH analysis of circulating tumor cells from 7 pts revealed MET copy number gain in 2 pts (28%). Six of 36 pts assessable for response including at least 3 pts with prior E therapy had вүҘ 30% reduction in tumor measurements on at least 1 post-baseline scan, including 3 with a cPR (1 with MET amplification). Prolonged SD вүҘ 4 months has been observed in some pts including one pt for 9+ months and one pt with EGFR T790M. Conclusions: The overall safety and tolerability profile of XL184 + E appears acceptable without evidence of a XL184/E DDI. Encouraging clinical activity of XL184 + E was observed in a largely E pretreated population, including pts with EGFR T790M and MET amplification.
|